N.D.C. BARBOSA, F. PIMENTEL-SOUZA and I.B.M. SAMPAIO*
Departamento de Fisiologia e Biofisica, Instituto de Ciências Biológicas and
*Departamento de Zootecnia, Escola de Veterinária, Universidade Federal de Minas Gerais, 30160-970 - Belo Horizon te, MG, Brasil
I. Reproduction in the snail Biomphalaria glabrata, a vector of Schistosoma mansoni, was measured by egg production and other parameters such as number of clutches, eggs per clutch and egg hatching, under laboratory conditions,
2. Reproductive rates were highest in terms of total number of eggs, clutches and hatching under constant illumination, followed by conditions similar to the natural rhythm of illumination. The greatest reduction in reproduction occurred in constant darkness. In summary, reproductive rate was dependent on both the intensity of absolute illumination and the schedule of illumimtion exposure,
3. Seasonal variation in reproductive rate appeared to vary inversely with local temperature in Belo Horizonte, situated in a tropical zone (latitude 20 S) owing probably to a temperature preference oi 20 to 25°C by the snail, The highest reproductive rates occurred in winter, except for the parameter eggs per clutch which was maximal in autumn. Snail reproduction appeared to be most reduced during summer. There was no linear correlation between reproductive rate and temperature.
Key words: Schistosoma mansoni vector, Biomphalaria glabrata, illumination schedule, season, temperature, reproductive rates, egg laying,
Introduction
The trematode infection, schistosomiasis, is estimated to affect more than 10 million Brazilians. One of the major strategies developed to combat the disease has been the elimination of the principal intermediate hosts of the parasite, such as the freshwater snail Biomphalaria glabrata. However, the reproductive biology of this snail is stiff unclear, Standen (1951) has suggested that egg production in B. glabrata is variable, as confirmed by Schall (1980), while Brumpt (1941) has suggested that greater fertility B. glabrata occurs under conditions of high illumination. Paulini and Camey (1964) have observed reduced egg production in darkness and Joy (1971) has shown that egg laying is constant in the light period regardless of the length of the light period in a day, decreasing in the dark period as the length of that period decreases.
Other known parameters most influencing egg production in snails are: water temperature, volume of the water available per snail and shell diameter. Growth rates affect age at sexual maturity and, thus, egg production (Pereira and Deslandes, 1964). Larger snails show higher rates of reproduction at an earlier age (Ritchie et at, 1966; Freitas et al., 1975 ; Scherrer et al., 1976; Gerken, 1977). Since larger snails may result from earlier oviposition, the parameter age, therefore, may be substituted by snail size. Snail density can be substituted by the volume of water available per snail, thus considering the crow ding effect (Chemin and Michelson, 195 7 ; El Hassan, 1974).
In view of the variable effect of light on egg production in Biomphalaria glabrata and its importance for the spread of schistosomiasis, the present study attempts to: 1) determine the influence of the intensity of absolute illumination and the schedule of illumination exposure on egg laying, and 2) measure variation in egg production during the year in terms of seasonal temperature variation, with the following parameters kept constant : water volume, snail shell diameter and illumination conditions.
Materials and Methods
Uninfected, melanic, adult snails, about 60 days old, supplied by the Schistosorniasis Research Unit were used. Batches of fifty snails from the same pond, with mean initial and final shell diameters of12.09 + 1.98 mm, and 13.97 ± 1.I I mm, respectively, were employed for each seasonal experiment. Snails were from stock bred for the past 25 years under laboratory conditions at the Schistosorniasis Research Unit (GIDE), Institute of Biological Sciences, Federal University of Minas Gerais (UFMG) (Freitas, 1973). Snails were fed fresh lettuce renewed daily ad libitum.
Twenty -five, 200 ml beakers, each containing two snails, were utilized for each seasonal experiment. Prior to use, water was filtered through fine sand and charcoal to eliminate chlorine and impurities. A layer of soil, consisting of a mixture of sterilized earth and lime (8 : 1) was placed in each beaker. Beakers were covered with transparent, plastic sheets (Souza and Paulini, 1967).
Five illumination conditions were used :
The artificial illumination consisted of fluorescent "daylight" lamps (Philips No. 54) providing an intensity of approximately 100 lux at the level of the beakers. In constant dark, illumination was 0.02 lux.
Water temperature varied little during the day (± 0.5°C). For each experimental phase, temperature was measured at about 9 :00 a-m- on the first and last days. Daffy variation during the year (from 19.5 to 26.3°C) was slow and dependent on chamber temperature inertia. Local air temperature was supplied by the Weather Forecasting Service of a Ministry of Agriculture Weather Station (Belo Horizonte) some 10 km distant.
Data were obtained from summer 1981 until spring 1982, the experiments being carried out in the middle of each season. In each series, groups of 10 snails (2 per beaker) were placed in each of the five illumination conditions, 4 days being allowed for adaptation. The subsequent 5 days were taken as the experimental period, at the end of which eggs were removed and counted, water changed and the snails shifted to another illumination condition where the same procedure was followed. This procedure was repeated until all groups had been exposed to the five illumination conditions (latin square design). The sequence of illumination conditions for each group was selected at random.
After removal, eggs were maintained in water and observed daffy until hatching. Artificial illumination was maintained at about 12 h L: 12 h D. Experiments were designed using the latin square method to correct variability among experimental groups (Barbosa, 1984). Data were analyzed statistically using a logarithmic transformation and analysis of variance (Snedecor and Cochran,1967). Significant differences between means were calculated using the minimum significant difference (MSD) test (P = 0.05) (Snedecor and Cochran, 1967).
Results
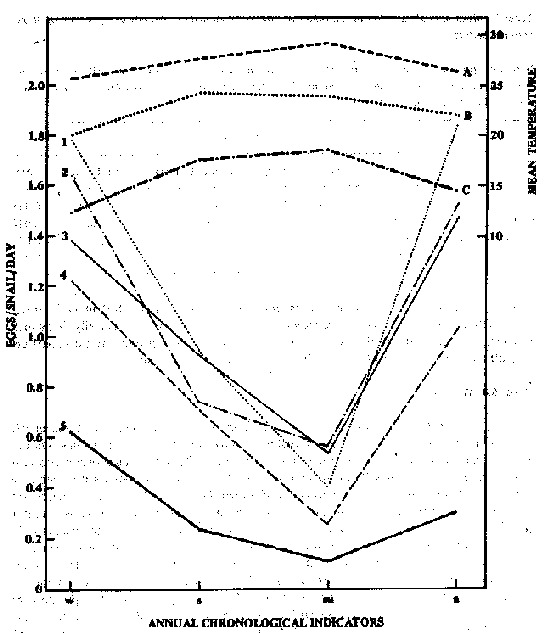
The reproductive rate of B. glabrata is best presented as eggs produced/snail/day. The highest mean seasonal production in the 5 illumination conditions was observed during winter, followed by autumn, spring and summer, all significantly different from each other (Table 1). However, to better control one of the independent variables (illumination) and demonstrate only the seasonal temperature effect, each illumination schedule was maintained constant during the year, as shown in Figure 1. Considering the different illumination conditions, the highest mean annual egg production/snail/day was seen in LL > 12 h L:12 h Dg > 12 h L:12 h Da > 4 h L:4 h D > DD, with the first and latter two schedule s differing significantly from the others (Table 2).
Table 1 . Variation in reproductive rates as a function of season in B. glabrata. Data are reported as means (with 99 degrees of freedom for the latin square design), All means within a given parameter are significantly different (P = 0.05, minimum significant difference test) except where indicatcd non-significant (NS), Several mean measures of temperature variation (°C) are provided.
Season |
||||
| Parameter | Summer | Autumn | Winter | Spring |
Eggs/snail/day |
1.863 | 6.185 | 6.633 | 3.537 |
Clutches/snail/day |
0.094 | 0.269 | 0.273 | 0.171 |
Eggs per clutch/snail/day |
0.416 | 0.612NS | 0.610 NS | 0.501 |
Hatching/snail/day |
1.195 | 3,895 | 4.857 | 1.937 |
Water temperature |
24.0 | 22,0 | 19.9 | 24.2 |
Air temperature |
23.8 | 20.4 | 19.6 | 22.0 |
Maximum air temperature |
29.0 | 26.2 | 26.7 | 26.6 |
Minimum air temperature |
18.5 | 14.5 | 12.4 | 17.5 |
Variation in clutch/snail/day was similar to that for egg/snail/day. During the experimental periods in the 5 illumination condftions, the highest mean number of clutches/snail/day was recorded in winter > autumn > spring > summer, each season differing significantly from the others (Table 1). However, to achieve better control of one of the independent variables (illumination); and to demonstrate only the seasonal temperature effect, each illumination scheduli'was maintained constant over the year, as shown in Figure 2. Considering the different illumination conditions, the highest mean annual number of clutches/snail/day was obtained in LL > 12 h L: 12 h Da > 12 h L : 12 h Dg > 4 h L: 4 h D > DD, with the first and latter two schedules differing significantly from the others (Table 2 ).

Figure 1 - Variation in mean number of eggs produced/snail/day and mean air and beaker water temperatures as a function of the period of the year under 5 illumination conditions: (1) continuous illumination; (2) 12 h Light : 12 h Dark gradual changes; (3) 12 h Light: 12 h Dark abrupt changes; (4) 4 h Light: 4 h Dark and (5) continuous darkness. A, mean maximum air temperature, B, mean water temperature, C, mean minimum air temperature; w, winter; s, spring; su, summer; a, autumn.
Variation in the number uf eggs per clutch/snail/day was also similar to that for egg/snail/day. Over the year under the 5 illumination conditions, the highest mean number of eggs per clutch/snail/day recorded was in autumn > winter > spring > summer, with the latter two seasons differing significantly from the former (Table 1). However, to better control one of the independent variables
Table 2 . Mean annual variation in reproductive rates in B. glabrata as a function of illumination schedules. Data are reported as means with 99 degrees of freedom for the latin square design. All means within a given parameter are significantly difiercnt (P =0.05, minimum significant difference test) except where indicated non-significant INS).
Illumination scheme |
|||||
| Parameter | LL | 12 hl:12 hDg | 12 hl:12hDa | 4hl:4hd | DD |
Eggs |
1.240 | 1.114 NS | 1.075 NS | 0.811 | 0.315 |
Clutches |
0.055 | 0.042 NS | 0.047 NS | 0.039 | 0.018 |
Eggs per clutch |
0.107 | 0.131 | 0.110 | 0.115 | 0.016 |
Hatching |
0.843 NS | 0.639 | 0.745 NS | 0.527 | 0.216 |
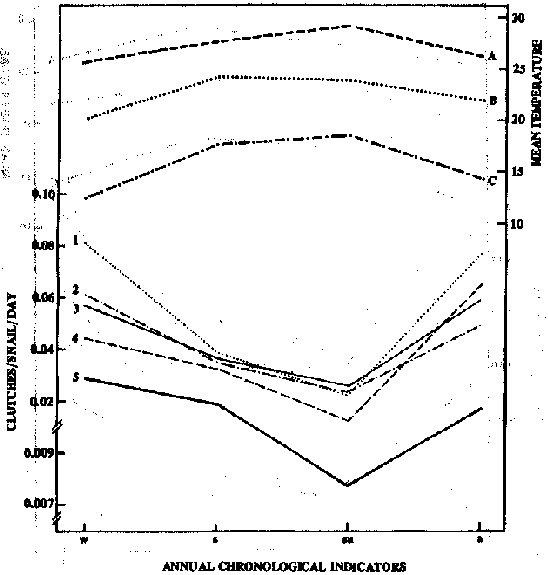
Figure 2 - Variation in the mean number of clutches produced/snail/day and mean air and beaker water temperatures, as a function of the period of the year, under 5 illumination conditions: (1) continuous illumination ; (2) 12 h Light: 12 h Dark, gradual changes; (3) 12 h Light: 12 h Dark, abrupt changes; (4) 4 h Light: 4 h Dark and (5) continuous darkness. A, mean maximum air temperature, B, mean water temperature, C, mean minimum air temperature ; w, winter; s, spring; su, summer and a, autumn.
(illumination) and to demonstrate only the seasonal temperature effect each illumination schedule was maintained constant over the year, as shown in Figure 3. Considering the illumination conditions, the highest mean annual number of eggs/ clutch was seen under 12 h L:12 h Dg > followed by 4 h L: 4 h D, 12 h L:12 h Da, LL and DD, each significantly different from the others (Table 2).
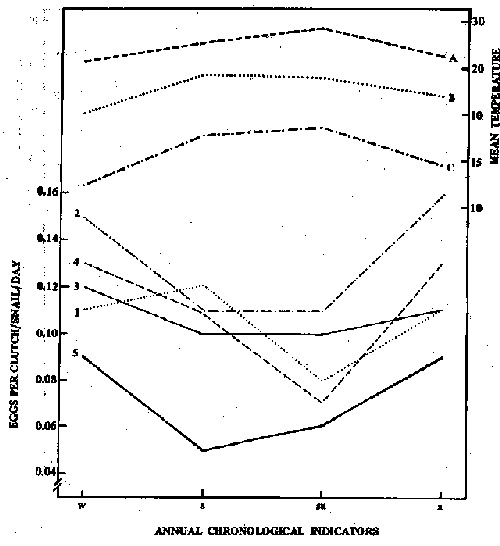
Figure 3 - Variation in mean number of eggs per cluch/snail/day and mean air and beaker water temperatures a function of the period of the year under 5 illumination conditions: (1) continuous illumination, (2) 12 h Light: 12 h Dark, gradual changes (3) 12 h Light: 12 h Dark, abrupt changes; (4) 4 h Light: 4 h Dark and (5) continuous darkness. A, mean maximum air temperature, B, mean water temperatures, C, mean minimum air temperature. w, winter ; s, spring ; su, summer and a, autumn.
Variation in the number of eggs hatching/snail/day was similar to that for eggs produced/snail/day. Over the experimental period in the 5 illumination conditions, the highest rate of hatching was found in winter > autumn > spring > summer, each significantly different from the others (Table 1). However, to better control one of the independent variables (illumination) and to demonstrate only the seasonal temperature effect, each illumination schedule was maintained constant over the year, as shown in Figure 4. Considering the illumination conditions, the highest mean annual hatching rates were seen in LL and 12 h L:12 h Da (not statistically different), followed by 12 h L:12 h Dg, 4 h L: 4 h D and DD, each significantly different from the others (Table 2).
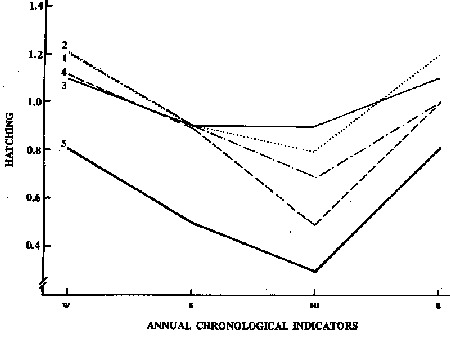
Figure 4 . Variation in number of eggs hatching/snaiiday under 5 illumination conditions:(1) continuous illumination, (2) 12 h Light: 12 h Dark, gradual changes, (3) 12 h Light: 12 h Dark, abrupt changes, (4) 4 h Light: 4 h Dark and (5) continuous darkness over the year. w, Winter; s, spring; su, summer and a, autumn.
Over the year, the reproductive rates of B, glabrata were highest in winter with the exception of the parameter eggs per clutch where the maximum was recorded in autumn although not significantly different from that for winter. The lowest count was found in summer, in this experiment, reproductive rate varied inversely with temperature although there was no linear correlation between the rates of reproduction and temperature, Illumination markedly increased rates of egg laying, clutches and hatching. The intensity of absolute illumination had less influence on eggs per clutch than on the other reproductive rate parameters. The schedule of illumination exposure, especially that which was similar to the natural diurnal rhythm, greatly influenced reproductive rates, particularly affecting the number of eggs produced, eggs per clutch and hatching.
Discussion
Standen (1951) found no evidence of fluctuation in egg laying in Biomphalaria glabrata, probably because his data consisted of only two records from a single season. Rey (1956) found clear seasonal variation in four species of Biomphalaria over a two-year experimental period in Brazil; an increase in'reproductive rate coincided with a decrease in mean air temperature in the experimental chamber, Gaud and Dupuy (1955) described a direct relationship between temperature and reproductive rate in another tropical vector of schistosomiasis, the snail Bulinus truncatus, where reproductive rate increased in warmer months over a two year period, in Morocco, The different relationships between reproductive rate and temperature in the above experiments are of little significance since the range of the experimental temperature must be correlated with the preferred temperature for egg laying, Thus, considering Brumpt's (1941) supposition that the optimum egg laying temperature for the genus Biomphalaria lies between 20 and 25°C, and extending tills hypothesis to the genus Bulinus, also a schistosomiasis vector, the maximum and minimum reproduction rates occwred at mean temperatures of 20,1 and 27.7°C, respectively, for Rey's (1956) experiments, and 24.2 and 13,7°C, respectively, for the genus Bulinus (Gaud and Dupuy, 1955). It thus seems reasonable to conclude that in Rey's experiments, 27.7°C was above, but 20,1°C, within the supposed optimal range ; in Gaud and Dupuy's experiments, 13,7°C was below, but 24,2°C within, the supposed optimal range for reproduction of the snails.
Field observations on the density of the snails confirm Brumpt's hypothesis: 1) the density of snails increases in winter in tropical zones such as Brazil (Andrade, 1962; Paulini and Camey, 1964; Barbosa, 1970, for Biomphalaria glabrata) and Egypt (El Hassan, 1974, for Biomphalaria alexandhna and Bulinus truncatus); 2) snail density increases in summer in temperate zones such as South Africa (Appleton, 1977, for Biomphalaria pfeifferi).
Latitudinal variation tllus results in temperature variation within and above or below the supposed optimum range of temperature for reproduction, modifying the relationships between temperature and reproduction from inverse dependence in the tropics to direct dependence in temperate zones.
The present data are consistent with Brumpt's hypothesis, taking geographical differences in temperature into consideration. However, under laboratory conditions, temperature variation was greatly attenuated throujh chamber inertia; the relationship between temperature and oviposition should be more evident under field conditions. Unfortunately, in field studies, methodical sampling cannot be employed throughout the year. Neither was it possible to employ a special design such as the latin square, to eliminate the effect of intrinsic variability in oviposition.
Szurnlewicz (1958) measured egg laying over a three year period in eleven generations of snails, in Rio de Janeiro, where local air temperature varied from 13 to 35°C. Kawazoe (1975) also measured egg laying at air temperatures from 14.8 to 34.7°C, in Campinas, Brazil. These two authors did not find clear seasonal variation in reproductive rate for the genus Biomphalaria under laboratory conditions probably because, although the optimum temperature for reproduction lay within the experimental temperature range, the real temperature of the snails in water under such conditions was greatly attenuated in relation to the air temperature and because the experimental design employed did not eliminate variability in oviposition.
In the present study, mean air and water temperature in autumn compared to winter, and water temperatures in summer compared to spring, were closer to the optimum range for oviposition predicted by Brumpt (1941) (Table 1). Thus, better rates of reproduction in autumn compared to winter would be expected, In fact, the predicted greater egg production occurred, although only in LL, The 4 h L:4 h D scheme is too different from the natural rhythm while DD produces a repressive state; emphasizing LL data, the reproductive rate in autumn was not higher in gradual or abrupt 12 h L: 12 h D conditions, The 12 h L:12 h Dg scheme was the only illumination condition lacking total control, given that oviposition remains constant regardless of day length (Joy, 1971). Thus, variation in the slow differential effect of light during illumination changes for 12 h L: 12 h Dg throughout the year must be considered, This appeared to be greater in winter than in autumn, and less in summer than in spring, These data are coherent with the physical effect of variation in sunshine at latitude 20 S in Belo Horizonte.
This effect of illumination on reproduction in the snail B. glabrata has not been previously studied, except for the confirmation of lower egg laying rates in darkness (Paulini and Camey, 1964) and Brumpt's (1941) suggestion that fertility should increase with increased light. The results of the present study indicate another important factor, i.e. illumination, to be considered in future laboratory experiments or field observations.
Egg production by B. glabrata varies widely, However, considering snails of similar shell diameter and the volume of water per snail, reproductive rates in terms of eggs produced/snail/ day (1.9 to 6.6) and clutches/snail/day (0.09 to 0.27) in the present experiments are similar to the data generally reported for Biomphalaria glabrata (Brumpt, 1941; Luttermoser, 1943; Standen, 1951; Rey, 1956; Szumlewicz, 1958; Paulini and Camey, 1964).
Egg hatching in B. glabrata is less variable. However, hatching in the present experiments (66 to 73%) was slightly less than the rates given by some authors (Brumpt, 1941; Szumlewicz, 1958; Sturrock and Sturrock, 1972; Freitas, 1973; Kawazoe, 1975 ; rated from 80 to 100fo), but similar to others (Penido et al., 1951; Rey, 195 6 ; Michelson, 1961; rated from 60 to 79%). In order to compare seasonal variation in hatching, especially due to temperature variation, illumination conditions must be controlled. However, the seasonal variation in reproductive rate of these snails, considered as the hatching of young snails, should depend more on the values for the other reproductive rate parameters than on hatching, owing to their wider range of values at roughly the same temperature.
Embryonic development, considered in the present study as the time from oviposition to hatching, varied from 9.6 to 14.7 days (mean, 12.2 days), with greater values in colder and lower values in warmer seasons. These data are coherent with those from the literature which vary from 2 to 30 days for Biomphalaria glabrata (Brumpt, 1941; Ripson, 1949 ; Penido et al., 1951; Rey, 1956; Lagrange, 195 7 ; Szumlewicz, 1958; Ritchie et al., 1966; Magalhães et al., 1968 ; Magalhães and Deluca, 1971; Sturrock and Sturrock, 1972).
References